核心提示: 近日,我校生命科学技术学院、农业微生物学国家重点实验室王革娇教授领导的环境微生物课题组在国际学术期刊Environmental Science and Technology发表了两篇关于微生物修复剂对多种重金属污染高效协同修复的最新研究成果,阐明了微生物菌剂通过新型氧化还原酶、琥珀酸与硫化氢代谢、生物膜合成等途径对重金属砷、铬、镉修复的分子机制。
南湖新闻网讯(通讯员 王杏 史凯祥)近日,我校生命科学技术学院、农业微生物学国家重点实验室王革娇教授领导的环境微生物课题组在国际学术期刊Environmental Science and Technology发表了两篇关于微生物修复剂对多种重金属污染高效协同修复的最新研究成果,阐明了微生物菌剂通过新型氧化还原酶、琥珀酸与硫化氢代谢、生物膜合成等途径对重金属砷、铬、镉修复的分子机制。
重金属砷(As)、镉(Cd)、铬(Cr)广泛分布于土壤环境中,重金属的过度积累能够引起土壤理化性质和生态结构的变化,并通过食物链进入人体,引起器官的病变,威胁民众健康。近年来农田土壤重金属污染修复成为国内外学者研究的热点。微生物参与了重金属的地球化学循环,然而如何通过微生物菌剂修复环境中的重金属以及相应的分子机制并不清楚。
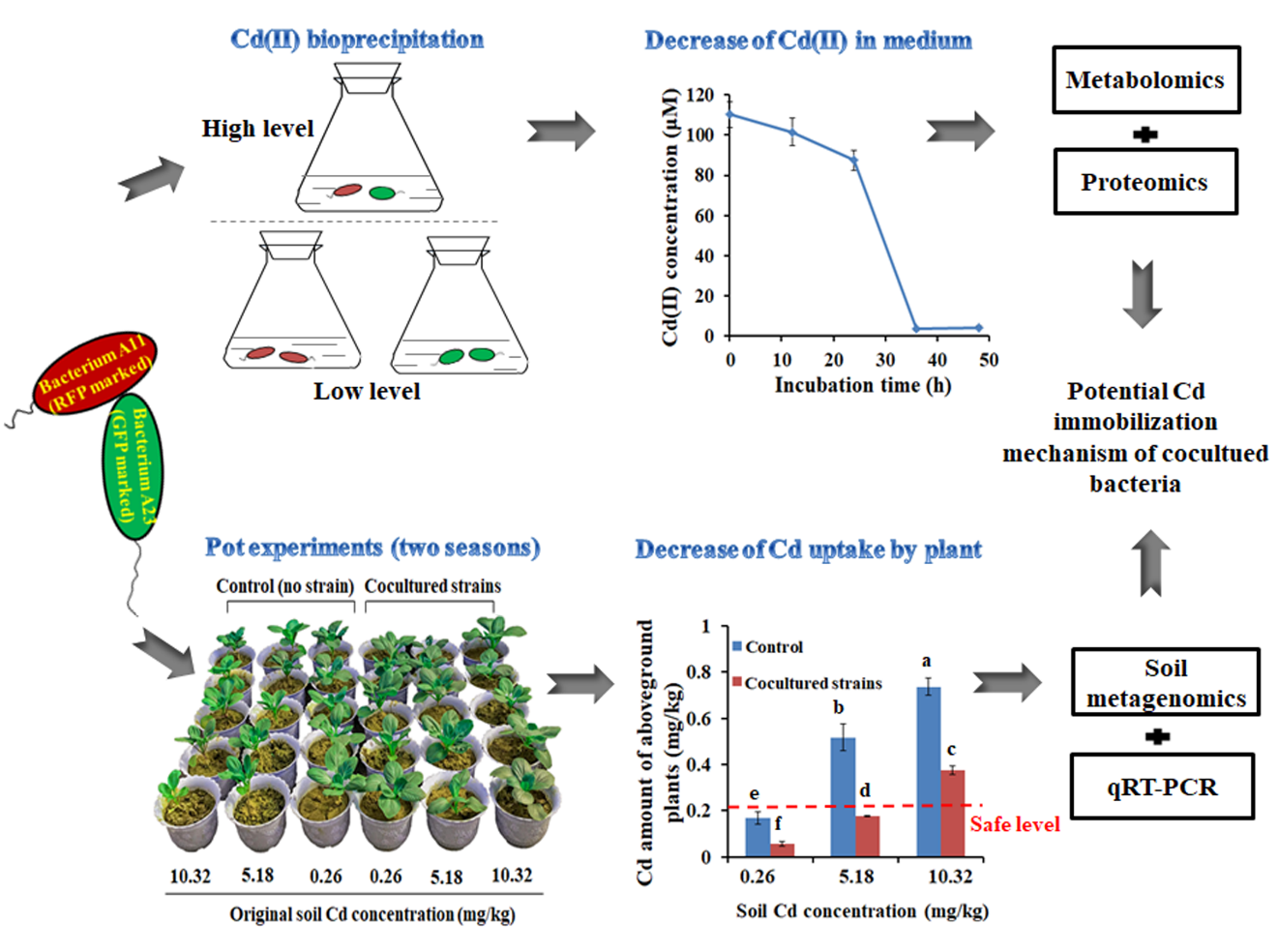
该课题组首先发现了能产硫化氢(H2S)的肠杆菌和产生物膜的丛毛单胞菌。两株细菌共同培养时,可以将溶液中的镉离子沉淀并完全去除。采用差异蛋白质组学和代谢组学分析技术,发现在镉胁迫下,共培养菌株中肠杆菌的H2S代谢、琥珀酸合成、金属离子转运和TCA循环等途径被激活。体外加入琥珀酸,促进肠杆菌产生更多的H2S,促进丛毛单胞菌产生更多的生物膜来固定镉离子,表明硫化氢、生物膜和琥珀酸是高效镉离子去除的重要因子。将这种混合菌剂应用于中度和高度镉污染白菜盆栽实验中,通过两季的重复实验,发现该混合菌剂可以有效的钝化土壤中的镉,减少植物对镉离子的吸收,使白菜地上部分镉含量分别下降了63%和39%,达到了安全可食用范围。采用荧光标记平板计数法、qRT-PCR及土壤宏基因组学分析,发现该混合菌剂可以在土壤中稳定的定殖并与土壤中的土著细菌有相互作用。
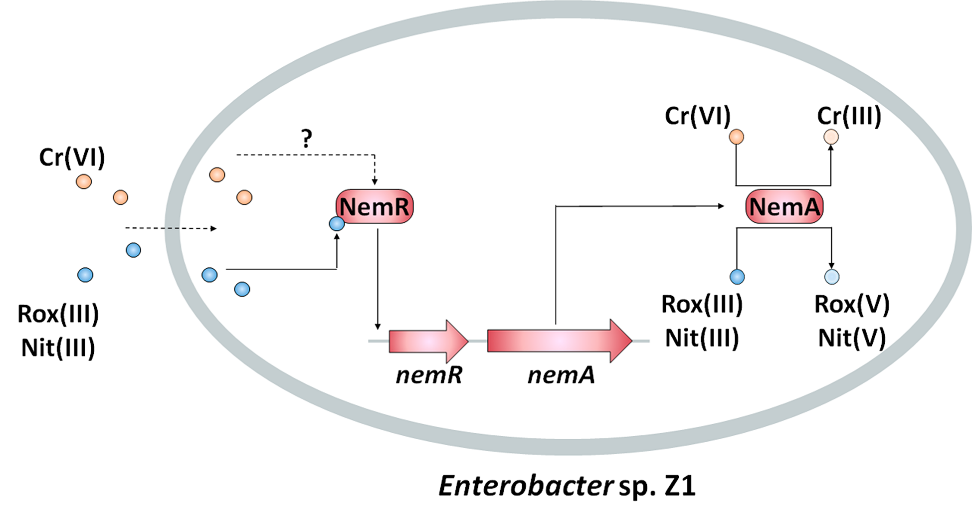
此外,该课题组通过基因组分析和基因转录检测等分子生物学手段发现了新型的氧化还原酶NemA受到有机三价砷及六价铬的诱导。通过异源表达实验以及体外酶活检测发现NemA能够提高大肠杆菌对有机三价砷及六价铬的抗性,且NemA能够将高毒性的有机三价砷氧化为低毒性的有机五价砷、将高毒性的六价铬还原为低毒性的三价铬。在砷与铬共同存在的条件下,NemA砷氧化速率具有显著的提升,表明NemA能够将电子从有机三价砷传递给六价铬,从而完成两种高毒性重金属的协同解毒。色氨酸荧光光谱及凝胶迁移率实验表明调控蛋白NemR能够结合有机三价砷,进而调控nemA基因的转录表达。通过对NemR蛋白晶体结构分析最终确定了21位、106位和116位半胱氨酸为NemR识别有机三价砷信号的保守位点。NemR为首次发现的对砷和铬同时解毒的酶,该酶在铬砷污染的生物修复方面具有开发潜力。
该课题组发现了一种新的细菌互作钝化镉离子的现象并阐明了其机制;鉴定到一种新型的广泛存在于细菌中的有机砷解毒系统,首次从遗传学和酶学角度探究了微生物中砷铬协同代谢机制。其研究结果为理解微生物驱动的环境中砷、铬、镉循环迈出了重要的一步,为微生物互作在镉污染修复中的重要作用揭开了新的一页,具有重要理论价值和应用潜力。
该研究工作得到国家十三五重点研发计划、国家自然科学基金及中国博士后科学基金的资助。
文章1标题: Immobilization of Cd using mixed Enterobacter and Comamonas bacterial reagents in pot experiments with Brassica rapa L
【英文摘要】Enterobacter sp. A11 and Comamonas sp. A23 were isolated and identified. Coculturing these two strains with Cd(II) led to the production of biofilm, H2S, and succinic acid (SA), and Cd(II) was adsorbed by cells and formed CdS precipitates. After centrifugation, 97% Cd(II) was removed from the coculture. Proteomic and metabolomic analyses of the cocultured bacteria revealed that H2S and SA production pathways, metal transportation, and TCA cycle were active under Cd(II) stress. In vitro addition of SA enhanced the production of H2S and biofilm formation and Cd(II) adsorption. Two-season greenhouse pot experiments with Brassica rapa L. were performed with and without the coculture bacteria. Compared with the control, the average Cd amounts of the two-season pot experiments of the aboveground plants were decreased by 71.3%, 62.8%, and 38.6%, and the nonbioavailable and immobilized Cd in the soils were increased by 211.8%, 213.4%, and 116.7%, for low-, medium-, and high- Cd-spiked soils, respectively. The two strains survived well in soil during plant growth using plate counting, quantitative real-time PCR, and metagenomics analysis. Our results indicate that the combination of Enterobacter and Comamonas strains with the production of H2S and biofilm are important effectors for the highly efficient immobilization of Cd.
论文链接:https://pubs.acs.org/doi/10.1021/acs.est.0c03114
文章2标题:NemA catalyzes trivalent organoarsenicals oxidation and is regulated by the trivalent organoarsenical-selective transcriptional repressor NemR
【英文摘要】Synthetic aromatic arsenicals such as roxarsone (Rox(V)) and nitarsone (Nit(V)) have been used as animal growth enhancers and herbicides. Microbes contribute to redox cycling between the relatively less toxic pentavalent and highly toxic trivalent arsenicals. In this study, we report the identification of nemRA operon from Enterobacter sp. Z1 and show that it is involved in trivalent organoarsenical oxidation. Expression of nemA is induced by chromate (Cr(VI)), Rox(III), and Nit(III). Heterologous expression of NemA in Escherichia coli confers resistance to Cr(VI), methylarsenite (MAs(III)), Rox(III), and Nit(III). Purified NemA catalyzes simultaneous Cr(VI) reduction and MAs(III)/Rox(III)/Nit(III) oxidation, and oxidation was enhanced in the presence of Cr(VI). The results of electrophoretic mobility shift assays and fluorescence assays demonstrate that the transcriptional repressor, NemR, binds to either Rox(III) or Nit(III). NemR has three conserved cysteine residues, Cys21, Cys106, and Cys116. Mutation of any of the three resulted in loss of response to Rox(III)/Nit(III), indicating that they form an Rox(III)/Nit(III) binding site. These results show that NemA is a novel trivalent organoarsenical oxidase that is regulated by the trivalent organoarsenical-selective repressor NemR. This discovery expands our knowledge of the molecular mechanisms of organoarsenical oxidation and provides a basis for studying the redox coupling of environmental toxic compounds.
论文链接:https://pubs.acs.org/doi/10.1021/acs.est.1c00574
审核人:王革娇
